Methane is one of the most widely used fuels in the world today, but its impact on climate change is forcing us to find alternatives to it, and hydrogen is hyped as its potential successor.
Both hydrogen and methane are highly efficient sources of energy, but they have several significant differences that make transitioning from methane to hydrogen challenging. In this article, we will explore hydrogen and methane from a chemical, energy, production, environmental, and safety perspective in order to understand how each fuel can be best utilized for its intended purpose.
By understanding these differences between hydrogen and methane we can begin to understand the downstream impacts on the transition pathways.
Chemical Makeup of Hydrogen and Methane
Hydrogen and methane are both chemical compounds that contain hydrogen. However, the two substances have different molecular structures. Hydrogen is composed of two hydrogen atoms bonded together (H2), while methane is composed of four hydrogen atoms and one carbon atom (CH4). This difference in molecular structure has implications for their energy content, production methods, and impact on climate change.
Just for a moment, let's go back to high school science and talk about the periodic table. Elements on the periodic table are arranged by atomic weight, and hydrogen, being the first element on the periodic table, is a very light element with an atomic weight of 1, and with two hydrogen atoms making up a hydrogen molecule, the molecular weight of hydrogen is 2. But when compared to methane, with a molecular weight of 16, it’s a heck of a lot lighter. In fact, it's about 8 times lighter than methane and about 14 times lighter than air meaning if it is released into the air, it will rise quickly.
Hydrogen is also the smallest atom known to humans, which means it also has the greatest potential to escape from its container (a gas bottle, pipe, etc.) and leak into the atmosphere. And with its ignition characteristics, this does cause some concerns.
Flammability range is a term used to describe the highest and lowest mixtures of a fuel with air at which it can be ignited. The wider this range is, the more likely it is that the fuel will be ignitable if it is present in the air, and the narrower, the less likely.
Hydrogen possesses a wide range of flammability, with the lower limit or lowest concentration at which it can ignite being 4.1% and the upper limit being 75%. Within the flammability range, there is an ideal point at which 'complete combustion' occurs, meaning that all of the fuel is consumed in the combustion process. For complete combustion, hydrogen should be mixed with air at around 30%.
Comparatively, methane possesses a flammability range, with the lower limit or lowest concentration at which it can ignite being 5% and the upper limit being 20%. Within the flammability range, there is an ideal point at which 'complete combustion' occurs, meaning that all of the fuel is consumed in the combustion process. For complete combustion, methane should be mixed with air at around 10%.
Hydrogen's increased flammability range makes it more dangerous when in an enclosed space such as a kitchen, or other rooms in homes, offices, and factories.
Energy Content/Density (Heating Value) of Hydrogen and Methane
Hydrogen and methane have significantly different energy contents, and which fuel looks better on paper depends on how you measure its energy content. Energy content can be expressed in energy per unit of weight (e.g. MJ/kg) or energy per unit of volume (e.g. MJ/m³).
Hydrogen has an energy content by weight of 142 MJ/kg and by volume of 12.8 MJ/m³, while methane's energy content by weight is 55.5 MJ/kg and by volume of 38 MJ/m³. So, if you consider energy content by weight, hydrogen looks better on paper. But by volume, methane wins out.
But why do these two comparisons provide such different outcomes? This all comes back to the atomic weight of hydrogen and methane. Because methane is 8 times heavier than hydrogen, the volume required to achieve 1 kg of it is a lot less. 1 kg of methane occupies about 1.4 m³ of volume, while 1 kg of hydrogen is about 11.1 m³.
To use the available energy, we need it in a gaseous state, and because hydrogens' energy content by volume is about a third of methanes, we need to consume three times as much of it to get the same output. This means we either need bigger pipes to transport it, or to put hydrogen under higher pressure to move it faster.
Hydrogen Flame Speed and Detection
When it comes to combustion, hydrogen has some unique properties that set it apart from other fuels. For one, hydrogen flames are almost invisible in daylight and can be difficult to detect with the naked eye. At night, hydrogen flames are visible, but they can still be hard to see compared to other fuels. To ensure the detection of hydrogen flames, thermal imaging cameras can be used.
Another unique aspect of hydrogen flames is that they emit substantial ultraviolet radiation but little infrared heat. This means that someone close to a hydrogen flame may not feel the heat, which can be a concern if they accidentally come into contact with the flame. Additionally, because of the emission of UV, prolonged exposure to hydrogen flames can result in sunburn-like effects.
Flame speed is a term used to describe how fast a flame travels through a mixture of unburned fuel and air mixture from the source of ignition. Hydrogen has a flame speed between 2 - 3m/second, whereas methane has a much slower flame speed, around 30-40cm/sec.
The impact of hydrogen flame speed on domestic gas appliances is also a concern as it can impact the safety and performance of these appliances. With a speed nearly 10 times faster than methane, it can be challenging to control the location of combustion in gas appliances, leading to potential issues such as oscillating combustion or flashback. These phenomena can occur when the flame moves upstream of the ideal combustion location, similar to a backfire in a car engine. To address these concerns, changes in the design of domestic gas appliances may be necessary to effectively manage the hydrogen flame speed and ensure safe and efficient operation.
Hydrogen Flame Temperatures
Hydrogen has a higher flame temperature compared to natural gas, reaching around 2,200°C (compared to 1,960°C for methane). This elevated temperature can pose a challenge for equipment and components, so it's important to carefully consider materials, cooling requirements, and the placement of temperature-sensitive components when using hydrogen as a fuel.
And although hydrogen helps reduce CO2 emissions, burning hydrogen can result in increased nitrogen oxides (NOx) emissions, as the higher flame temperature and the amount of nitrogen in the air contribute to NOx production which also has negative environmental impacts. Now NOx emissions can be reduced by controlling the air-to-fuel ratio during combustion, controlling flame hot spots, and using emission treatment systems.
Production Methods for Hydrogen and Methane
Hydrogen and methane are both produced through different methods. Hydrogen can be produced using a variety of methods including steam reforming of natural gas, thermochemical water electrolysis, or biological processes such as photosynthesis or microbial methanogenesis.
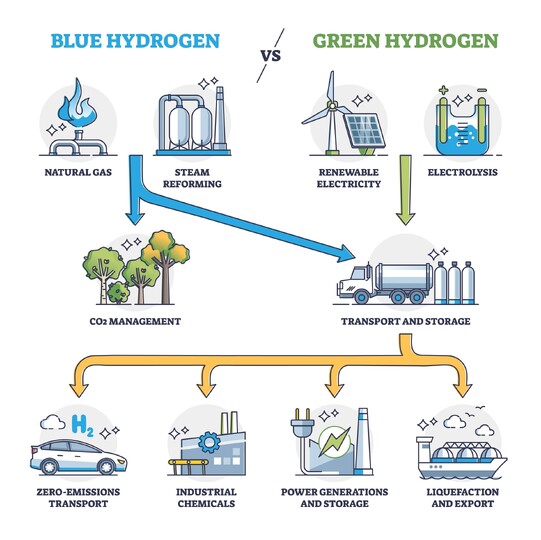
Steam methane reforming is the most commonly used method for producing hydrogen, where natural gas (methane) is converted into carbon dioxide and hydrogen by passing it over a catalyst at high temperatures. However, this process releases carbon into the atmosphere which, to fight climate change, we're trying to avoid. Again, consuming pure hydrogen is carbon-free, but if we can't get hydrogen without releasing carbon, we're no better off.
Hydrogen gas can be produced by thermochemical water electrolysis, a process that employs an electrolyzer powered by electricity to break down water molecules into oxygen and hydrogen. While this process is energy intensive and currently cost-prohibitive when compared to steam methane reforming, the ability for it to be powered by renewable energy rather than methane means that, if hydrogen is to be one of our clean energy sources in the future, electrolysis will be the way we access it.
Methane, on the other hand, can be produced from a variety of sources including fossil fuels like coal and oil, or biogenic sources such as biomass or landfill gas. Fossil fuel sources account for the majority of global methane production with approximately 79% coming from this source in 2015 according to the EIA. Biomass sources such as agricultural residues or municipal waste are also increasingly being used to produce methane in anaerobic digesters which convert organic matter into methane-rich biogas. Landfill gas is also a source of methane that is generated naturally as organic matter decomposes in landfills over time.
Environmental Impact of Hydrogen and Methane
The environmental impacts of hydrogen and methane depend largely on how they are produced and consumed. When looking at greenhouse gas emissions, both fuels can have an effect, but the magnitude of their respective impacts can be different depending on the production method involved.
For example, producing hydrogen from renewable energy sources like solar power has no direct emissions of greenhouse gases; however, if hydrogen is produced from natural gas through steam reforming, it will result in carbon dioxide emissions (remembering that methane is made up of four hydrogen atoms and one carbon atom).
Methane's direct emissions depend on the source of its production as well. Emissions from natural gas production tend to be lower than those from coal-based production due to differences in combustion efficiency; conversely, biogenic sources such as landfill gas or biogas have virtually no direct emissions due to their renewable nature.
However, when hydrogen is consumed there are no carbon emissions because hydrogen is a pure molecule. Whereas methane contains hydrogen and carbon, meaning that when it is consumed it releases carbon dioxide into the atmosphere.
Closing Thoughts
It is clear that we need to move away from methane as a fuel source if we are going to reduce climate change. While in a vacuum, hydrogen looks great on paper as a replacement fuel source, the pathway to transitioning from methane to hydrogen is complex. Irrespective of pathways, this transition will take decades to implement.